ABSTRACT
BACKGROUND/PURPOSE: There is currently no standard effective treatment for patients with relapsed/refractory primary central nervous system lymphoma (PCNSL). The prognosis is poor, so more studies are needed. Pemetrexed is similar to methotrexate in structure and can cross the blood-brain barrier. Compared with methotrexate, it has more targets and fewer side effects. Lenalidomide is a second generation immunomodulator with multiple functions, such as regulating immunity, anti-tumor and tumor microenvironment. A prospective observational study was conducted to explore the efficacy and safety of pemetrexed combined with lenalidomide in the treatment of relapsed/refractory primary central nervous system lymphoma.
METHODS: This is a prospective observational study, we collected patients who had undergone whole brain radiotherapy and two or more chemotherapy regimens between January 2018 and December 2019 but still experienced disease progression or recurrence. each patient was treated with pemetrexed at a dose of 900mg/m2 and lenalidomide 25mg over 3 weeks as salvage chemotherapy, and one cycle consists of 4 weeks. Folic acid, vitamin B12 and dexamethasone were used to induce toxicities to pemetrexed given before chemotherapy. Oral aspirin was used to prevent thrombosis caused by lenalidomide. Adverse events were recorded in each patient during inpatients, outpatient or telephone follow-up.
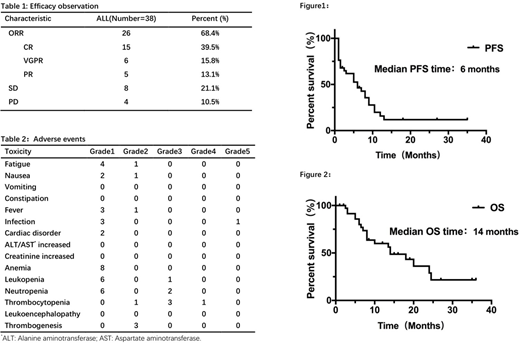
RESULTS: A total of 38 patients with recurrent/refractory PCNSL were enrolled in our study, including 26 males and 12 females with a median age of 57 (range from 33 to 73) years old. After treatment, the overall response rate was 68.4%(Table 1). The median progression free survival (PFS) time was 6 months and median overall survival (OS) time was 14 months (Figure1, Figure2). The adverse events mainly included fatigue, gastrointestinal reaction, myelosuppression, thrombocytopenia, fever and infection. After long-term or short-term chemotherapy, the patients had different degrees of myelosuppression symptoms presented as leukopenia (six cases in grade 1 and one case in grade 3), neutropenia (six cases in grade 1 and two cases in grade 3), anemia (6 cases in grade 1), thrombocytopenia (1 case in grade 2 and 3 cases in grade 3). Nausea and vomiting, as the common gastrointestinal reaction, appeared in two (grade 1) and one (grade 2) cases, respectively. One patient died of severe pneumonia infection. Three cases developed grade 1 cardiac disorder, including asymptomatic sinus bradycardia and asymptomatic ventricular premature beat, respectively. In addition, other reactions including fatigue (four cases in grade 1 and one case in grade 2), fever (four cases). All patients had no abnormal liver function, kidney function, constipation and leukoencephalopathy (Table 2).
CONCLUSION: This study has been the first prospective clinical study of pemetrexed combined with lenalidomide in the treatment of patients with relapsed/refractory PCNSL in international. The results indicate that chemotherapy with pemetrexed combined with lenalidomide may be an effective therapy for the treatment of relapsed/refractory PCNSL with modest toxicity.
KEYWORDS: Primary central nervous system lymphoma; Relapse/refractory; Pemetrexed combined with lenalidomide; Efficacy; Safety
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal